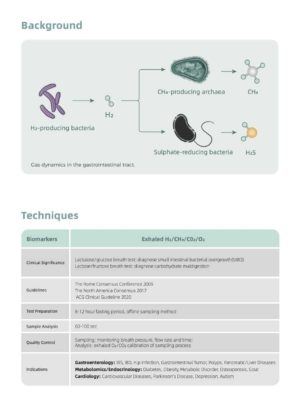
CA2122 measures Nitric Oxide (NO) in human breath. Nitric Oxide is frequently increased in some inflammatory processes such as asthma. The fractional NO concentration in expired breath (FeNO), can be measured by CA2122 according to guidelines for NO measurement established by the American Thoracic Society.
Measurement of FeNO by CA2122 is a quantitative, non-invasive, simple and safe method to measure the decrease in FeNO concentration in asthma patients that often occurs after treatment with anti-inflammatory pharmacological therapy, as an indication of the therapeutic effect in patients with elevated FeNO levels.
CA2122 is suitable for children, approximately 0 – 17 years, and adults 18 years and older. The device can be used with infants or by children under age of 7, or any patient who cannot cooperate with any necessary requirements of test performance, or in critical care, emergency care or in anaesthesiology by novel Offline/Tidal Sampling Modes.
FeNO measurements provide the physician with means of evaluating an asthma patients response to anti-inflammatory therapy, as an adjunct to the established clinical and laboratory assessments in asthma. CA2122 also measures Carbon Monoxide (CO) which is a biomarker for smoke cessation recommended by NICE guidelines.
CE marked In vitro diagnostic medical device for professional use.
Manufacturer: Sunvou Medical Electronics Co., Ltd.