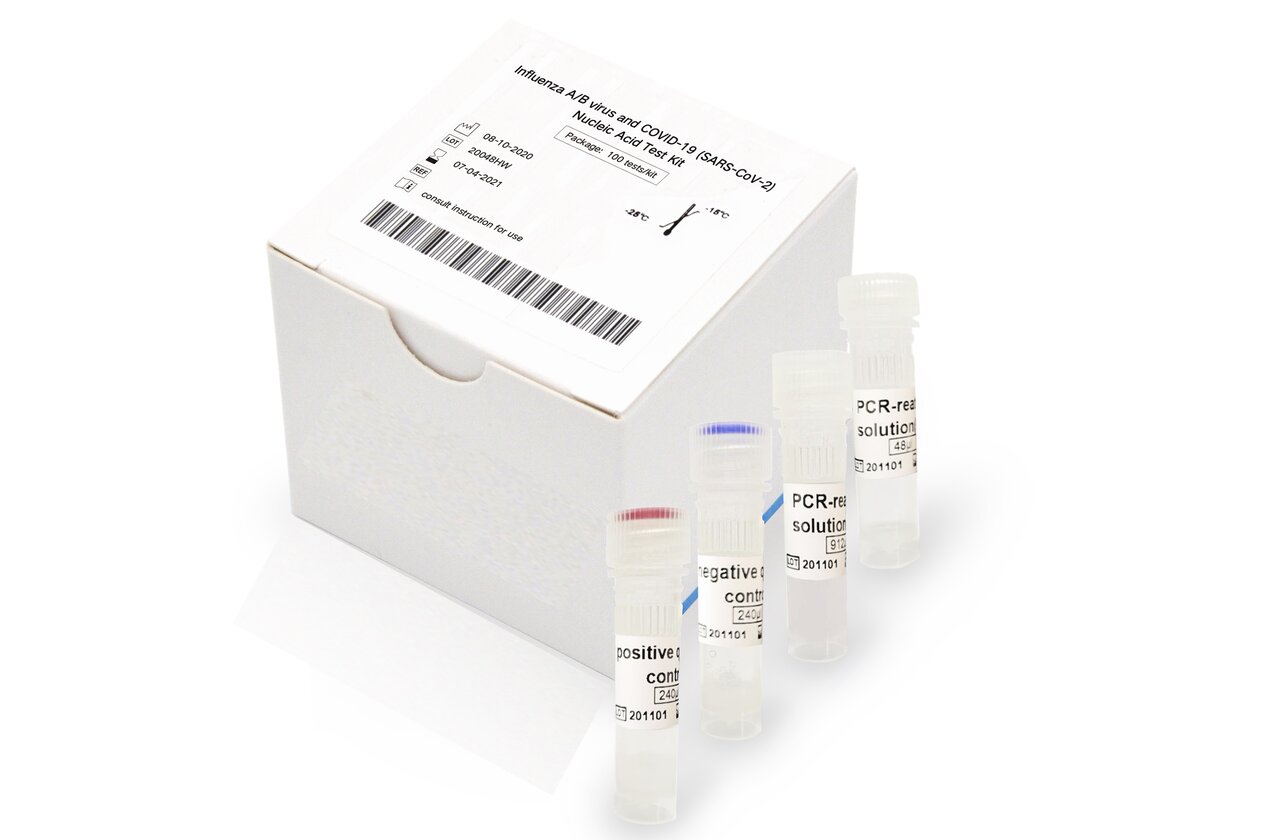
COVID-19 & Influenza A/B Nucleic Acid Test Kit
CE marked In vitro diagnostic medical device for professional use.
Manufacturer: Wuhan EasyDiagnosis Biomedicine Co., Ltd.
The kit is used for in vitro qualitative test of influenza A virus M gene, influenza B virus NS gene and 2019-nCoV (SARS-CoV-2) ORF1ab gene in suspected cases infected by influenza A/B virus and 2019-nCoV (SARS-CoV-2).
The test results of the kit are for clinical reference only and shall not be used as the only standard for clinical diagnosis, it is recommended to conduct a comprehensive analysis of the patient’s condition in combination with clinical manifestations and other laboratory tests.
The kit applies multiple PCR-fluorescent probe technique for test. It is combined with one-step RT-PCR technique, respectively takes the specificity conserved sequence of 2019-nCoV (SARS-CoV-2) ORF1ab gene, Influenza A virus (FA) M gene and Influenza B virus (FB) NS gene as target region, designed with target gene detection primer probe and equipped with RT-PCR reaction system for testing. After reaction, judge the results by analyzing the cycle threshold value (Ct value) of every channel. In addition, the kit is designed with the human housekeeping gene RNaseP as internal standard for monitoring, sampling, extracting, adding specimen and amplification to effectively prevent the occurrence of false positives.